Oxbridge-Science-Academy.github.io
Dipoles and Infrared Spectroscopy
So far we have assumed that all bonds can absorb infrared radiation. For simplicity, we shall only consider diatomic molecules in the following discussion. There is a criterion which much be satisfied for a bond to absorb to infrared radiation (called being IR active).
A bond will absorb infrared radiation if there is a change in the dipole moment during the vibration.
The theory behind this is beyond the scope of the course but we can still use this statement to predict whether a molecule will be IR active. The only potentially unfamiliar term is “dipole moment”. A dipole refers to an electric dipole which arises when there is a separation of positive and negative charge. The separated charges need not be full charges and dipoles arise when there is an electronegativity difference between the atoms of a diatomic molecule. The more electronegative element will have a partial negative charge δ− and the less electronegative element a partial positive charge δ+. The partial charges are fractions of the electronic charge 𝑒. The charge is typically denoted 𝑞.
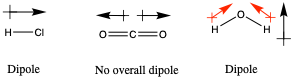
Dipoles are typically indicated on molecule by drawing an arrow in the direction a test positive charge would move. For example, consider H-Cl; H is δ+ and Cl is δ−. A test positive charge would move from the hydrogen to the chlorine. It can be helpfully to start the arrow by drawing a ‘+’ sign and then drawing the arrow from this to remind yourself that is is the direction a positive charge would move.
This can be done with other more complicated molecules like CO2 as shown. To find the total dipole of a molecule, we simply add up all the arrows, treating them like vectors. For example if they point in opposite direction they will cancel each other out. This is the case for CO2 where there is no total dipole so the stretch where both bonds lengthen or shorten at the same time is IR inactive as there is no change in the dipole (it remains zero).
Water has a dipole because Oxygen is more electrongative than Hydrogen. Note that in this case the net dipole does not point along the bonds but rather directly from the Hydrogens to the Oxygen. This is becasue the net dipole is the sum of the two individual dipoles (as in CO2) shown in red and we can see that the horizontal components of these red dipoles cancel and only the vertical component remains.
Change in dipole
However, as the statement in bold above says, we are actually concerned with a change in the dipole, not whether this an existing dipole. This is an important difference and is best understood by first considering the mathematical expression for a dipole moment.
The dipole moment 𝜇 is the product of the charge 𝑞 (partial or full) and its separation 𝑟:
𝜇=𝑞𝑟
The bond length will vary during vibration and this will change the separation, 𝑟, causing the dipole to change.
We only need consider 3 points during the bond vibration. These points are minimum length (maximum compression) 𝑟=𝑟_𝑚𝑖𝑛, maximum length (maximum extension) 𝑟=𝑟_𝑚𝑎𝑥 and the equilibrium length 𝑟=𝑟_𝑒𝑞.
At minimum length 𝜇=𝑞𝑟=𝑞𝑟_𝑚𝑖𝑛=𝜇_𝑚𝑖𝑛
At maximum length 𝜇=𝑞𝑟=𝑞𝑟_𝑚𝑎𝑥=𝜇_𝑚𝑎𝑥
At equilibrium length 𝜇=𝑞𝑟=𝑞𝑟_𝑒𝑞=𝜇_𝑒𝑞
𝜇_𝑚𝑖𝑛<𝜇_𝑒𝑞<𝜇_𝑚𝑎𝑥
Therefore, during the course of one vibration of the bond, the dipole changes and the bond can absorb IR radiation.
A diatomic molecule will be IR active if it is possesses a permanent dipole.
It should be stressed that the general statement on the previous slide applies to vibrations in all molecules, diatomic or not, but the statement above applies to diatomic molecules.