Oxbridge-Science-Academy.github.io
13C NMR
Coupling in 13C NMR tends to occur only between adjacent nuclei.
13C has an abundance of ~0.1% or 1 in every 1000 carbon atoms. The remaining 99.9% is almost entirely NMR inactive 12C. This has implications for 13C NMR spectra. The probability of finding two 13C nuclei adjacent to each other is in the region of ~10-3 times that of just finding one 13C. Therefore the signal from any such 13C- 13C coupling will be tiny compared to the signal generated by a 13C nucleus not engaging in coupling and hard to see.
However a 13C nucleus will couple to any hydrogen nuclei to which it is bonded. For example, a 13C in CH4 will produce a quintet by coupling to 4 1H nuclei. While this can provide helpful information about the numbers of hydrogens bonded to a particular carbon, in reality it complicates the spectrum significantly as carbons are often bonded to multiple protons and display multiplets. Peaks from different carbon environments can overlap, making spectrum analysis hard. To deal with this, a process called proton decoupling is applied.
Proton decoupling involves the application of a secondary band of radio frequencies to the sample which causes the proton nuclei to flip between the ↓ and ↑ spin states very rapidly such that the average spin tends to zero. The 13C nucleus does not observe a particular spin on the 1H nuclei and so no coupling occurs.
Other types of coupling can be applied to remove the coupling between 13C and other NMR active like 19F.
By applying decoupling, each carbon environment will produce a single, sharply defined peak. We can therefore immediately determine the number of carbon environments from the number of peaks in a 13C spectrum. The position of a peak in the spectrum, quantified by its chemical shift value δ, provides information about the local environment of the corresponding carbon. While there will always be exceptions, a general rule is:
δ=0−50 ppm sp3 C
δ=50−100 ppm sp3 C with electronegative element attached
δ=100−150 ppm sp2 C
δ=150−220 ppm sp2 C with electronegative element attached
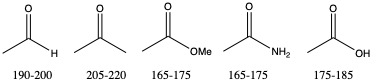
Just like IR spectroscopy, different carbonyls can be distinguished by the chemical shifts on the carbonyl carbon. All carbonyl carbons are sp2 and have at least on electronegative element attached (carbonyl oxygen) so fall in the δ=150−220 ppm region.
Conjugated rings like benzene can have a range of δ values depending on heteroatoms and substituents. δ tends to fall in the region 110−170 ppm.
1H NMR
First and foremost, 1H NMR spectra indicate the number of different proton environments in a molecule. However, they are usually more complicated than proton-decoupled 13C spectra since we do not remove the effects of coupling. As previously discussed, the splitting patterns of protons can become very complicated due to multiple non-equivalent environments.
However, information from the coupling patterns can help with structure determination by telling us the relative proximity of particular protons.
Like carbons, the chemical shift value of a proton provides information about its chemical environment. However, unlike carbon signals which have a range of 0-200 ppm, proton signals have a much smaller range of 0-10 ppm and the signal from a proton in a particular chemical environment (e.g. O-H proton) can vary by several ppm. This means it is much harder to assign a proton to a particular chemical environment just by looking at its chemical shift. Consideration of its splitting pattern and coupling constants is vital.
The chemical shifts of a some common proton environments are listed below:
Aldehydes δ ~10 ppm
Carboxylic Acid (O-H) δ=10−11 ppm
Amides (N-H) δ=5−9 ppm
Amines (N-H) δ=1−5 ppm
Benzene / Aromatic (C-H) δ=6−8 ppm (Look for multiple peaks clustered together)
Alcohol (O-H) δ=1−4.5 ppm
sp3 carbon δ=0.9−2 ppm (Increased by adjacent electronegative atoms)
sp2 carbon δ=5−5.3 ppm (Increased by adjacent electronegative atoms)
The overlap of the alcohol proton and sp3 carbon ranges show that chemical shift values alone are not normally enough to assign a proton to a particular environment.
Symmetry and NMR Environments
The symmetry of a molecule must be considered when determining the number of chemical environments. If two atoms in a molecule are related by a symmetry operation, e.g. reflection in a plane, they will be in the same environment. A common area of misunderstanding involves the convention for drawing benzene rings. You would be forgiven for initially thinking the two circled atoms are not equivalent since the green circled atom bonds via a double bond to the carbon on its right and the red circled atom does the opposite. However, while the 3 localised bonds drawing is preferred in academia as it makes drawing mechanisms easier, we must remember that all 6 carbons are equivalent as the 6 electrons are delocalised around the 𝜋 system (as shown by the lower diagram). Therefore, the circled atoms are equivalent. Linear molecules can also contain symmetry as illustrated by the lowest diagram. The different coloured circles show the 3 different chemical environments.